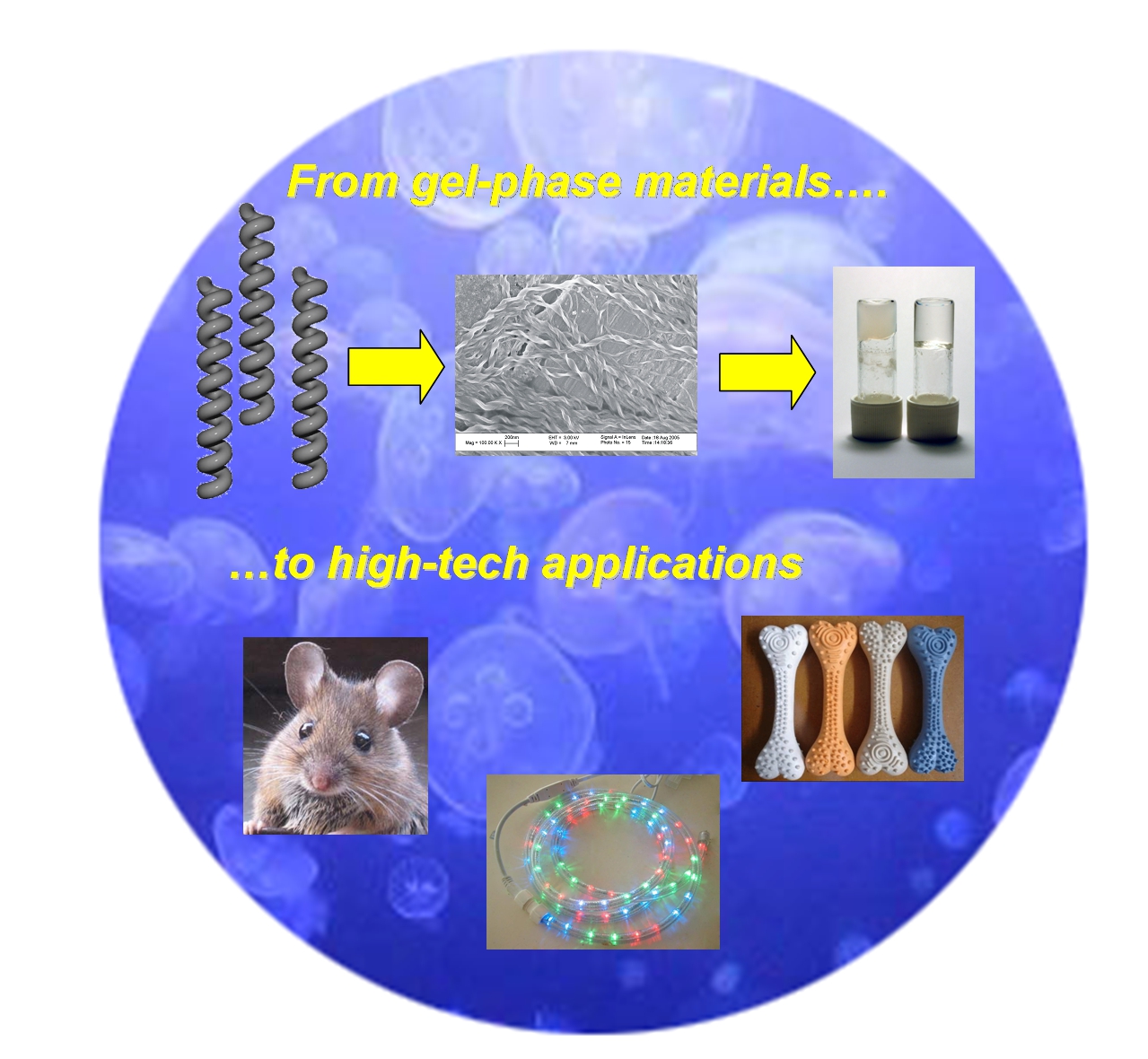
We are exploring one of the most exciting frontiers of modern chemistry - the nanoworld. Advances in microscopy mean that we can now easily see objects as small as just a few nanometres in size. Nanochemistry - the synthesis and study of nanoscale architectures, is a fundamental part of nanotechnology, as both materials and biological applications will depend on the new structures which chemists can generate. Our approach harnesses non-covalent interactions between molecules - supramolecular chemistry - in order to allow simple molecular-scale building blocks to spontaneously self-assemble into nanostructures. Self-assembly is a powerful and easy approach to constructing the nanoworld, and it allows us to generate a wide variety of systems, with applications ranging from nanomaterials to nanomedicine.
This page aims to summarise some of our major breakthroughs, and most influential research - you can connect to the original research articles via the embedded weblinks. For a complete Smith-group publication list, please use the 'publications' page. I hope you enjoy exploring some of our recent research.Gels are fascinating colloidal materials which surround us in everyday life - from Brylcreem Hairgel to Haribo Starmix. Most of the gels we see in everyday life are made of polymers. These large molecules form a 'solid-like' network within a 'liquid-like' solvent - this mix of solid/liquid properties gives rise to the soft solid properties of a gel with which we are all familiar. We are interested, however, in gels which form from small molecules, rather than polymers. The only way this can occur is through self-assembly, in which the molecules interact with one another to form nanofibres held together by non-covalent interactions. The key advantages of these 'supramolecular gels' is that they are highly tunable - by designing different functionalities into the molecules we can ensure that these functions become embedded within the gel-type material once assembly has taken place. Furthermore, because these gels are held together by non-covalent interactions - they are highly dynamic and responsive. This means that gels of this type have been suggested for a vast array of high-tech applications - ranging from nanoscale electronics to tissue engineering. We wrote a highly influential review on these applications Angew. Chem. Int. Ed. 2008, 47, 8002-8018.
 |
|
Supramolecular Gels with High-Tech Applications - From Tissue Engineering to Nanoscale Electronics
|
|---|
We entered the field of gel chemistry completely by chance in 2000, when we mixed two simple molecules together (a dendritic peptide and a diamine) and they formed a gel. We worked hard to understand why this took place, and in 2006, published a feature article in Chemical Communications which explained how the two components assembled into a dumbbell shaped complex, which could then assemble further into supramolecular polymer nanofibres in organic solvents as a consequence of hydrogen bond interactions between the peptide units. Chem. Commun. 2006, 34-44.
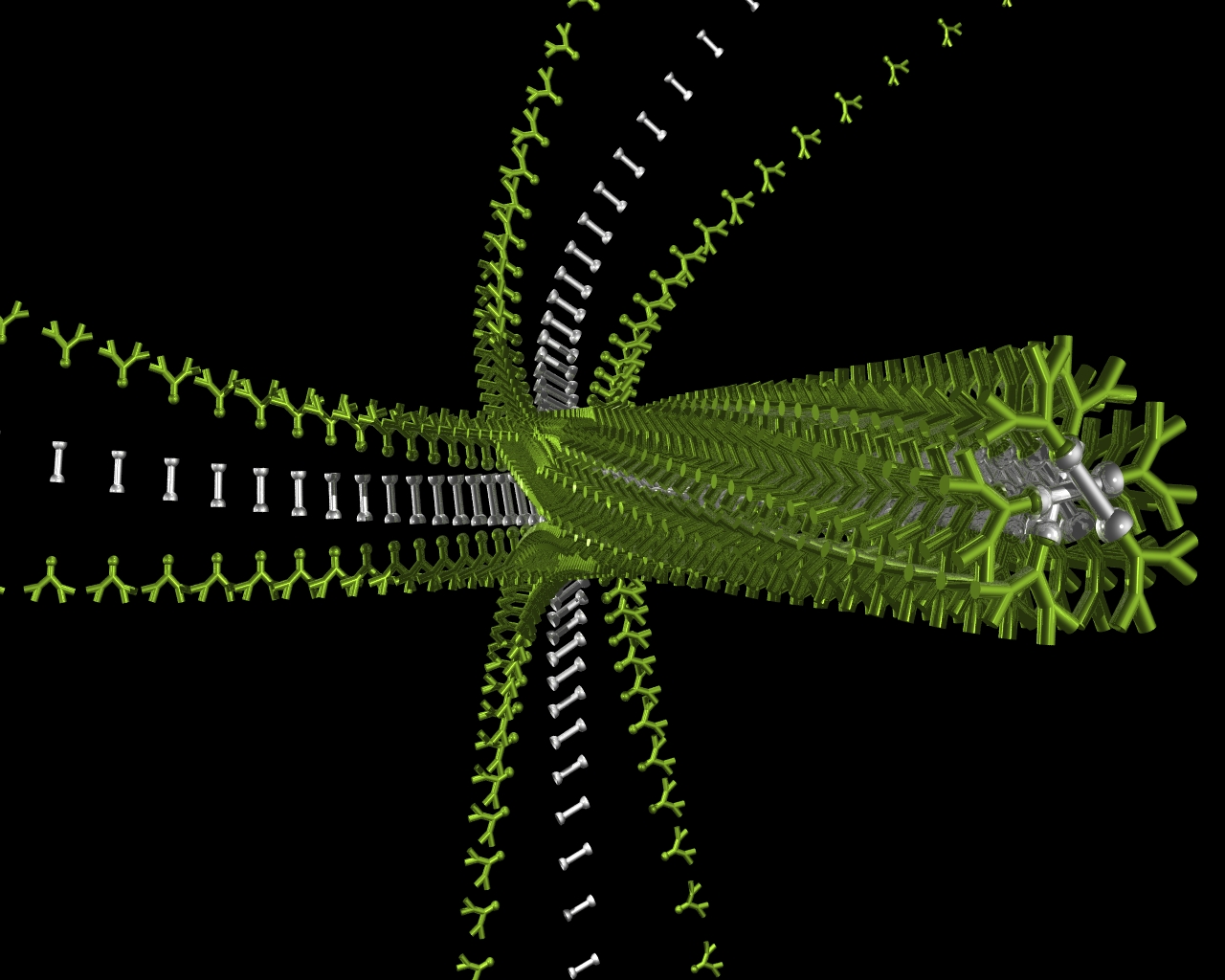 |
|
Supramolecuar Gels Assemble into Nanoscale Fibres from Molecular-Scale Building Blocks
|
|---|
Since this early work, we have aimed to generate fundamental understanding of many aspects gel-forming processes. This has led us to a number of novel insights into gelation, for example:
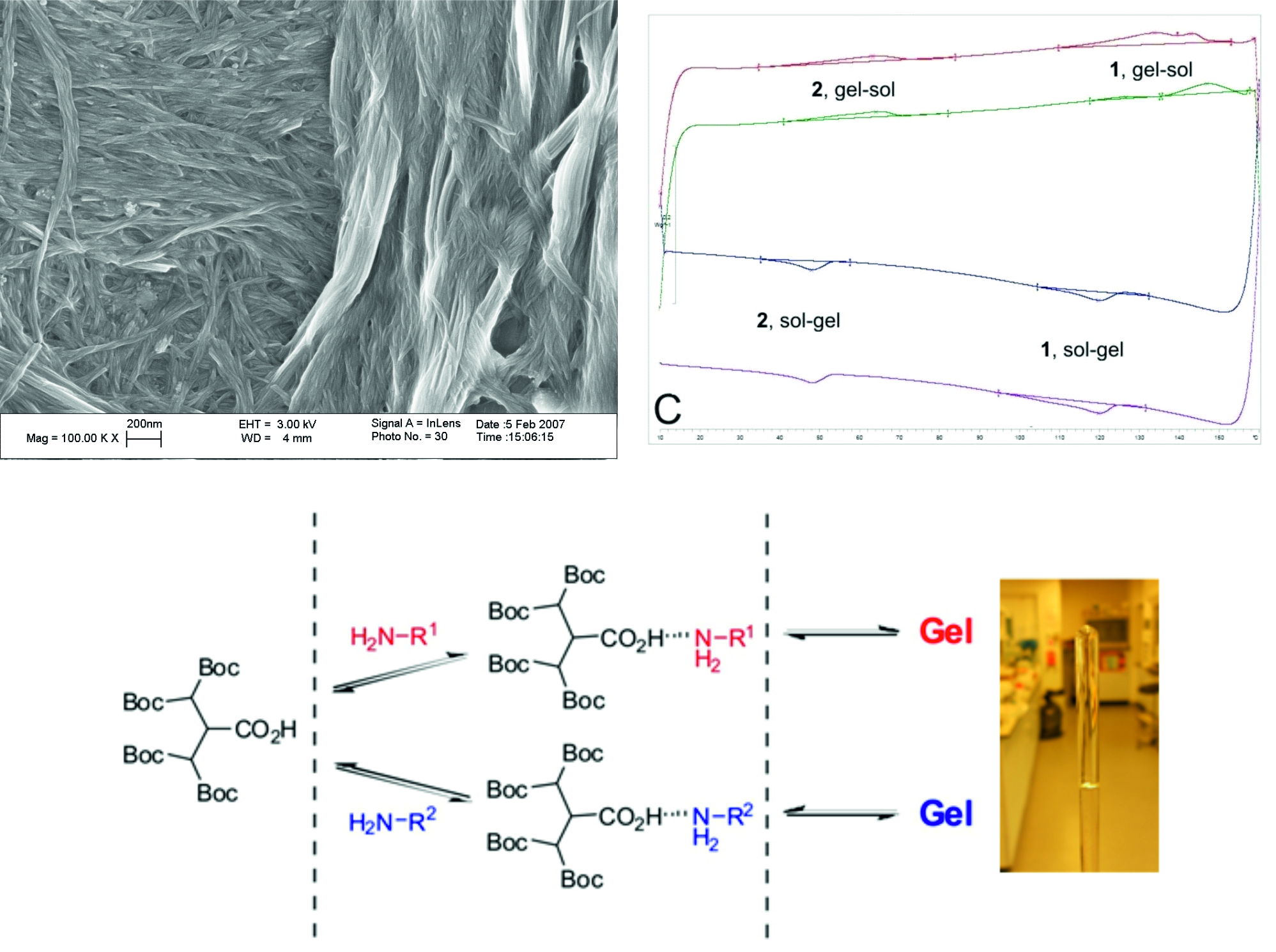
(i) Gels are dynamic materials that can be understood using NMR methods which allow quantification of what is in 'solid-like' and 'liquid-like' environments. This enables a full thermodynamic understanding of gelation as shown in our most highly cited research paper on gels: J. Am. Chem. Soc. 2008, 130, 9113-9121.
(ii) Gels are not always underpinned by one-dimensional supramolecular nanofibres - sometimes self-assembled two-dimensional platelets can form a 'solid-like' network. Chem. Eur. J. 2005, 11, 6552-6559.
(iii) Gels are metastable materials and can evolve further on standing - either into crystals Chem. Commun. 2008, 2248-2250 or into even better gels Soft Matter 2011, 7, 4856-4860.
(iv) The way in which a gel is formed can modify the way it self-assembles - we presented one example in which using slow cooling or fast cooling of a hot solution led to nanofibres or nanospheres respectively Chem. Eur. J. 2009, 15, 372-379.
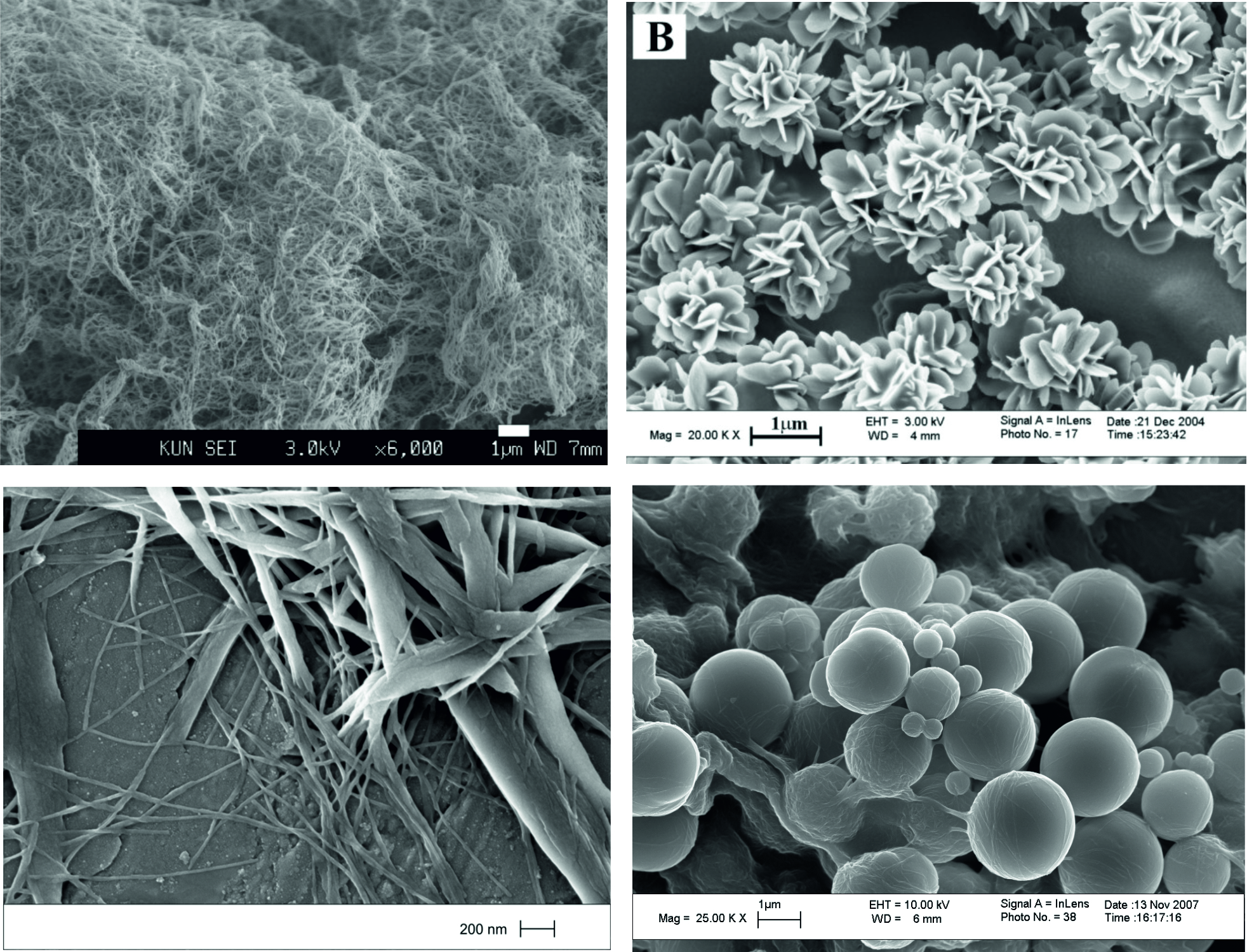 |
|
Self-Assembled Gels can have a Wide Range of Nanoscale Morphologies
|
|---|
In recent years we have become interested, some would say obsessed, with how gels can assemble from very complex mixtures of molecules. Does the molecular-scale information programmed into gelators still allow them to self-assemble when a chemical soup of different molecules is present? What about if the molecules are similar? Or different? If very different molecules are present, is each capable of assembling into its own gel nanostructure? Can we make multi-component multi-molecular gels with multiple functions?
We believe this is interesting because it demonstrates how molecular-scale engineering, combined with smart self-assembly can yield intelligent nanomaterials. Furthermore, it might mimic things that happen on the early earth, when order, and ultimately cellular life, had to emerge from complex chemical mixtures. Our key contributions to complex multi-component gel assembly have been:
(i) Learning which molecular-structural features control self-sorting of molecules into their own individual gel nanostructures. Chem. Eur. J. 2007, 13, 2180-2188.
(ii) Capturing the first electron micrsocopy image of a multi-gelator gel in which two different gelators self-sorted into their own nanostructures - one with thick fibres and one with thin fibres. Chem. Commun. 2009, 316-318
(iii) Showing that two different gel networks within a multi-gelator gel could be independently thermally addressable Soft Matter 2011, 7, 4856-4860.
(iv) Using NMR to quantify precisely which molecules are incorporated into gels assembled from highly complex environments, gaining a mechanistic understanding of how self sorting occurs, and importantly showing that these dynamic materials adapt and respond when exposed to new ingredients, and can heal their structures. J. Am. Chem. Soc. 2013, DOI 10.1021/ja4017107.
 |
|
Supramolecular Gels can be Assembled in Controlled Ways from Complex Mixtures Controlled by Non-Covalent Interactions
|
|---|
However, whilst doing all this fundamental research, we have not neglected the possible applications of these materials, and have published work on:
(i) The incorporation of nanogel networks into polymer films to enhance their materials performance (application: paints/plastics) Chem. Commun. 2008, 4601-4603.
(ii) Polymerising one of the gel networks in our self-sorted materials, washing away the second gel network, and leaving a nano-imprinted material with high swellability and solvent adsorbance capacity Langmuir 2009, 25, 8786-8793.
(iii) Generating two-faced polymers with embedded fluorescent nanofibres for use in nano-optics Chem. Commun. 2011, 47, 11864-11866.
(iv) Using nanofibres to organise arrays of gold nanoparticles for use in nano-electronics J. Mater. Chem. 2010, 20, 6696-6672.
(v) Silver responsive gels for use as nano-sensors Chem. Commun. 2012, 48, 2767-2769.
(vi) Several more pending applications ranging from environmental science to the lubrication industry.
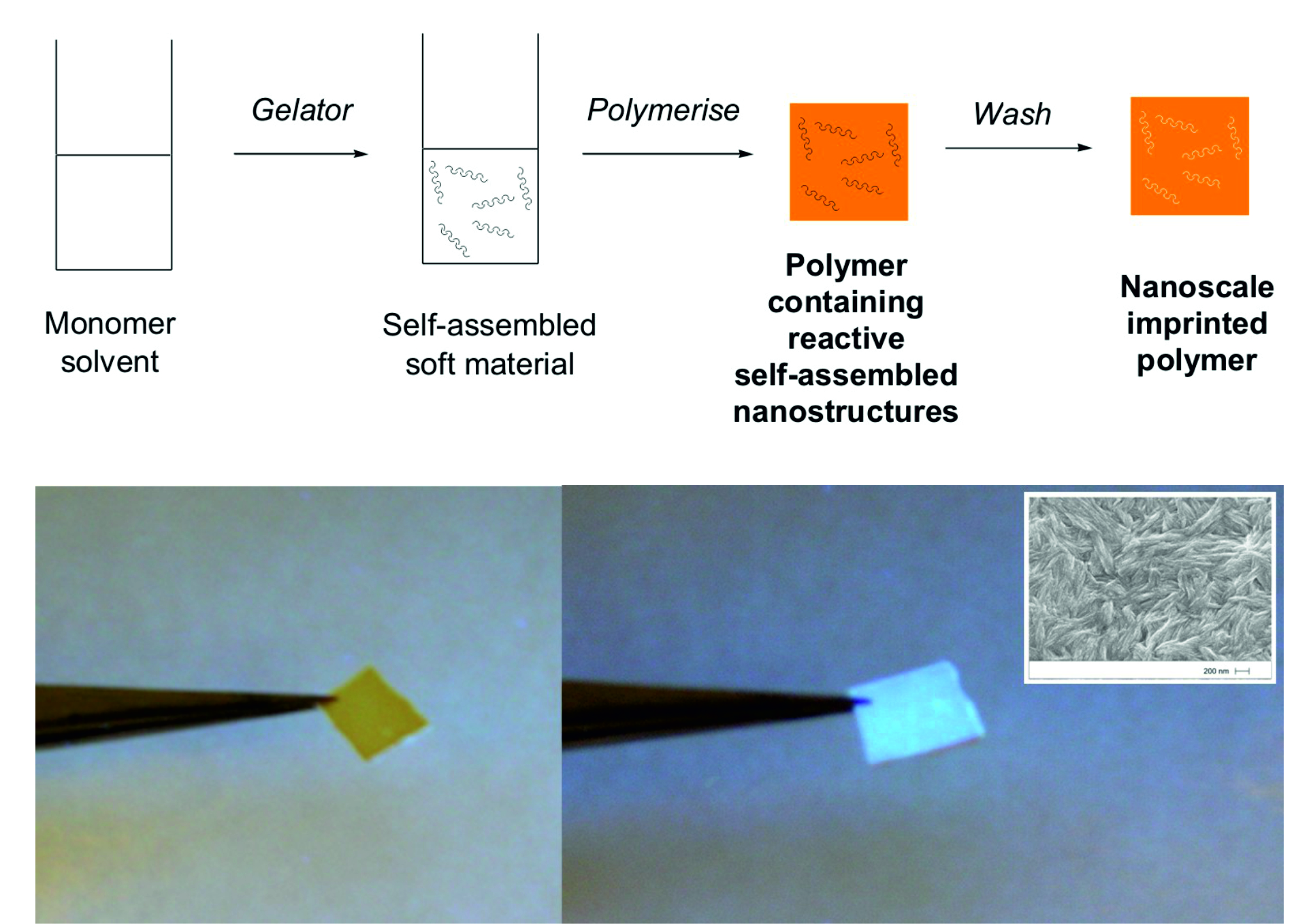 |
|
Supramolecular Gels can be Embedded into Materials to give them Novel and Enhanced Properties
|
|---|
Biological molecules such as DNA and proteins exist on the nanometre length scale, and as such, chemists wanting to intervene in biological processes are increasingly realising that forming interactions with such biomolecules on the nanoscale may be of great therapeutic value. However, although the nanoworld may seem incredibly small from the human viewpoint, from the point of view of a molecule, it is a pretty large place (a single bond is just 0.15 nm) - as such, we need to find effective ways of achieving high-affinity binding to nanoscale surfaces. One such strategy is multivalency - in which multiple ligands are used to interact with the bio-target. This is well-known to lead to much higher affinity binding because if one ligand becomes detached, there are plenty of others present to compensate.
Our interests in this area started in response to Dave's partner having cystic fibrosis, a genetic disease which leads to faulty chloride transport proteins, and ultimately, untimely death. One way of potentially treating diseases of this type is via gene therapy - delivering healthy genetic material into a patient's cells. However, in order to do this, effective vectors are required, which can bind and protect DNA, transport it into cells and allow it to be expressed and 'cure' the cell. This is a highly challenging target, and the focus of much work worldwide.
|
|
|
Gene Therapy - Delivering Genetic Material into Cells - Can be used to Treat Diseases from Cystic Fibrosis to Cancer
|
|---|
Our earliest work focused on the development of simple dendrons with spermine surface groups. Spermine is a known naturally-occurring DNA binder present in human sperm, and we reasoned that using it as the ligand on a multivalent branched (dendritic) array would allow us to achieve ultra high-affinity DNA binding. Highlights of our early work with this system included:
(i) The first reports of multivalent spermine and a demonstration that it achieved ultra-high-affinity salt-independent multivalent binding. Angew. Chem. Int. Ed. 2005, 44, 2556-2559
(ii) Molecular dynamics modelling to understand the origins of salt-independent binding, and the realisation that the flexibility of the dendron array enhanced mutivalency - a new paradigm in multivalent recognition, published in a high-impact paper. J. Am. Chem. Soc. 2009, 131, 9686-9694
(iii) The use of these arrays to functionalise proteins, which could as a result, achieve high-affinity adhesion to DNA. ACS Nano 2007, 1, 103-113.
(iv) The development of degradable systems in which multivalent binding could effectively be 'switched-off' by chemical degradation. Angew. Chem. Int. Ed. 2009, 48, 4047-4051.
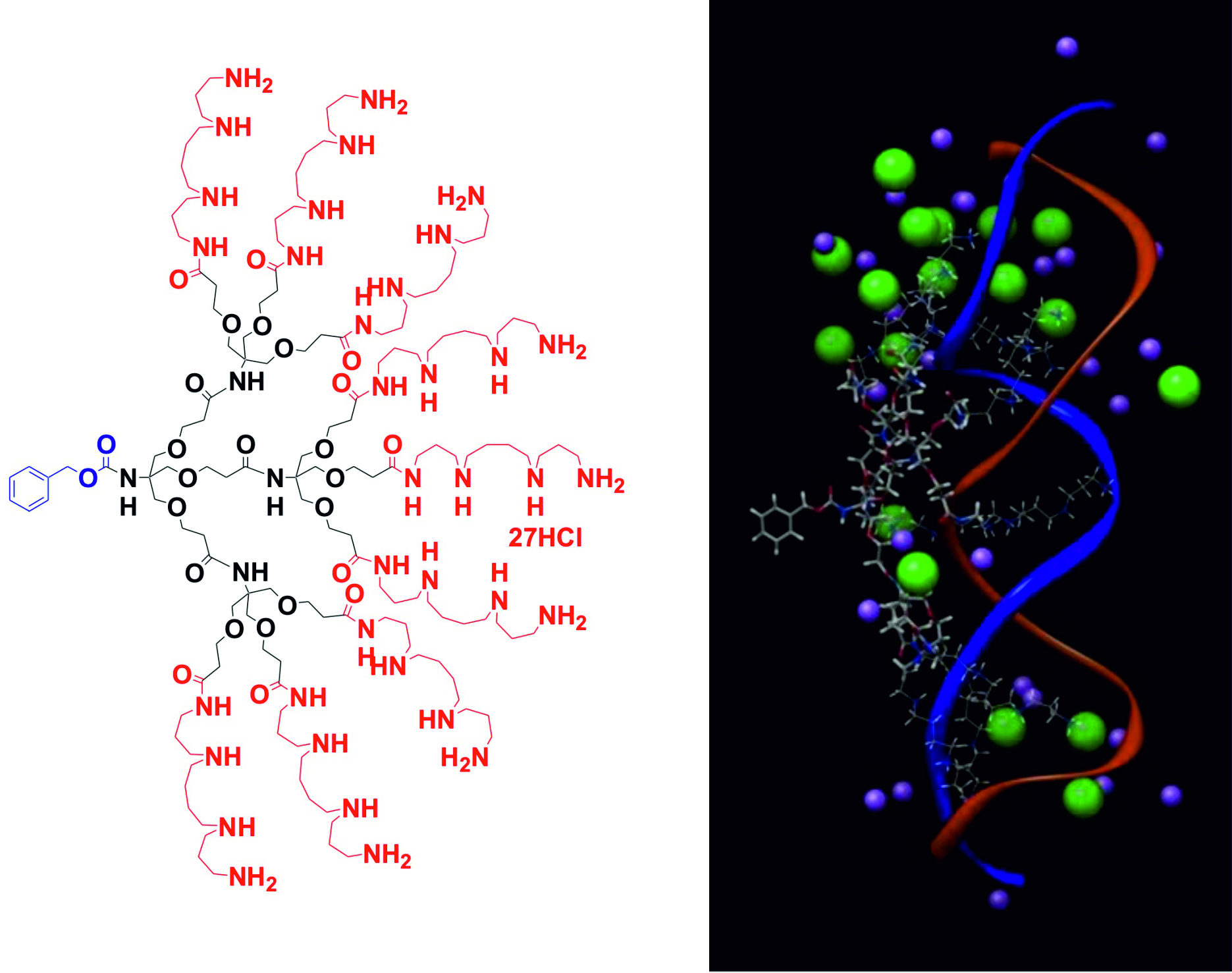 |
|
Multivalent Dendron for DNA Binding and Molecular Dynamics Model of Flexible Ligands Interacting with DNA
|
|---|
However, synthesising large branched arrays of ligands was time-consuming and challenging, and we wanted to simplify our approach. Learning from our work on nanomaterials, we thought that self-assembly might help us to multiple up small building blocks into larger ligand arrays, and as such, we decided to develop the concept of self-assembled multivalency, which was also beginning to be used in other labs. In this approach, self-assembly spontaneously generates a multivalent array, which can then achieve high-affinity binding. We wrote the first review article on this emerging topic, which is becoming highly influential Angew. Chem. Int. Ed. 2012, 51, 6572-6581.
As a consequence we began to exploit the use of Self-Assembled Multivalency (SAMul) in binding DNA and in combination with detailed multiscale modelling with our collaborators in Italy found the following key conclusions:
(i) We could use hydrophobic self-assembly in the core of our structures to control the surface density of our ligands and hence their DNA binding affinity, Mol. Pharm. 2011, 8, 416-429.
(ii) We could generate systems which were programmed to assemble into different morphologies, and in this way optimise the ability to deliver genetic material into cells. Chem. Commun. 2008, 4700-4702. and Chem. Sci. 2010, 1, 393-400.
(iii) Self-assembly provides a unique approach in which multiple active units can be simlpy mixed together into a single self-assembled structure, leading to easy tuning and optimisation. Org. Biomol. Chem. 2012, 10, 8403-8409.
(iv) With our degradable self-assembled multivalent DNA binding systems, we could fully understand every step in the gene delivery pathway - intriguingly binding to DNA prevented the system from degrading. J. Am. Chem. Soc. 2011, 133, 20288-20300.
|
|
|
Self-Assembled Multivalent Nanosystem Bound to DNA
|
|---|
We have learnt from these studies, and are now trying to develop simple self-assembling gene delivery vehicles with the correct profile of multi-functional activity potential clinical applications.
We have also demonstrated the principles of self-assembled multivalency using Arg-Gly-Asp peptides, that are known to target certain types of tumour cell which have integrin proteins in their membranes. We have demonstrated that:
(i) A self-assembled multivalent array is at least as good, if not better, in terms of integrin binding as displaying the peptides on a more traditional covalent scaffold. Org. Biomol. Chem. 2011, 9, 4795-4801.
(ii) The morphology of the self-assembled multivalent peptide arrays directly control the ability to bind to integrins. Org. Biomol. Chem. 2013, DOI 10.1039/C3OB00034F.
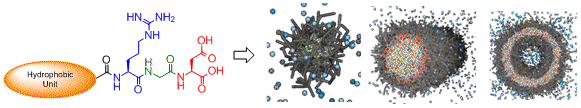 |
|
Multivalent RGD Nanostructures which undergo Controlled Self-Assembly into Different Morphologies
|
|---|
Heparin is, like DNA, also a nanoscale negatively charged polymeric biomolecule - specifically a sulfated polysaccharide. We became interested in binding heparin because it plays a vital role in major surgery - a fact we only realised when Dave's partner underwent a major operation. Heparin acts as an anti-coagulant during surgery - it would be highly desirable during major surgery for the anaesthetist to be able to monitor heparin-levels in real-time at the bedside, and as such, heparin sensors are of great interest. Furthermore, once surgery is complete, it is necessary to remove heparin from the patient's bloodstream, so that clotting can begin. The current agent, protamine, is not ideal, as a significant number of patients have adverse reactions to it - as such, designing new heparin rescue agents is also of great interest.
We have only been involved in this field since 2011, but it is proving to be very exciting. So far, our key breakthroughs include:
(i) The development of a novel heparin sensing dye called Mallard Blue, which binds to heparin through multiple electrostatic and hydrogen bond interactions, and can operate in human serum, and may offer a simple approach for detecting heparin levels. We named the dye as it has the same colour as the record-breaking steam locomotive 'Mallard', which can be found in the National Railway Museum in York - we believe that our dye is similarly ground-breaking in its performance as the Mallard. J. Am. Chem. Soc. 2013, 135, 2911-2914.
(ii) A self-assembled multivalent system which mimics the size, structure and surface charge of protamine and can bind to heparin with ultra-high affinity - this nanosystem may have applications as a protamine replacement. Angew. Chem. Int. Ed. 2011, 50, 4675-4679.
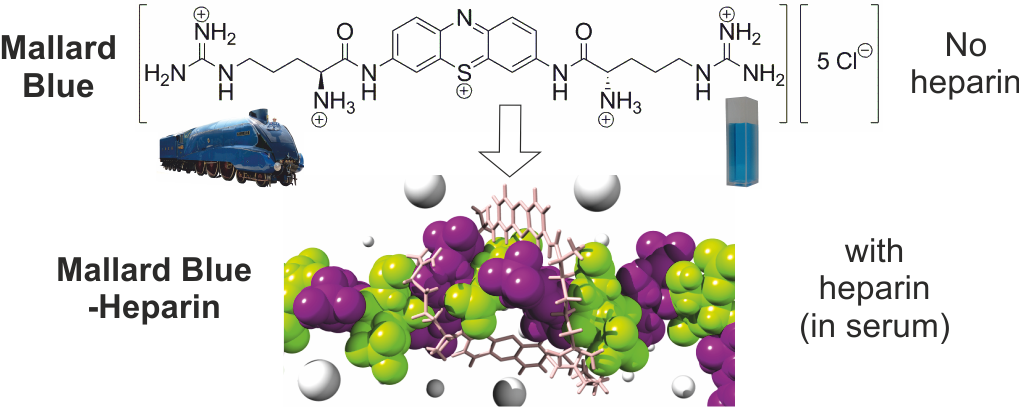 |
|
Mallard Blue - A Sensor for Heparin which works in Human Serum by Forming Multivalent Interactions as shown by Modelling
|
|---|