Research
| Transition Metal Nucleobases Complexes | Low-coordinate Phosphorus Chemistry |
| Carbon Monoxide-Releasing Molecules | Reactions of Alkyne Complexes |
Transition Metal Nucleobase Complexes
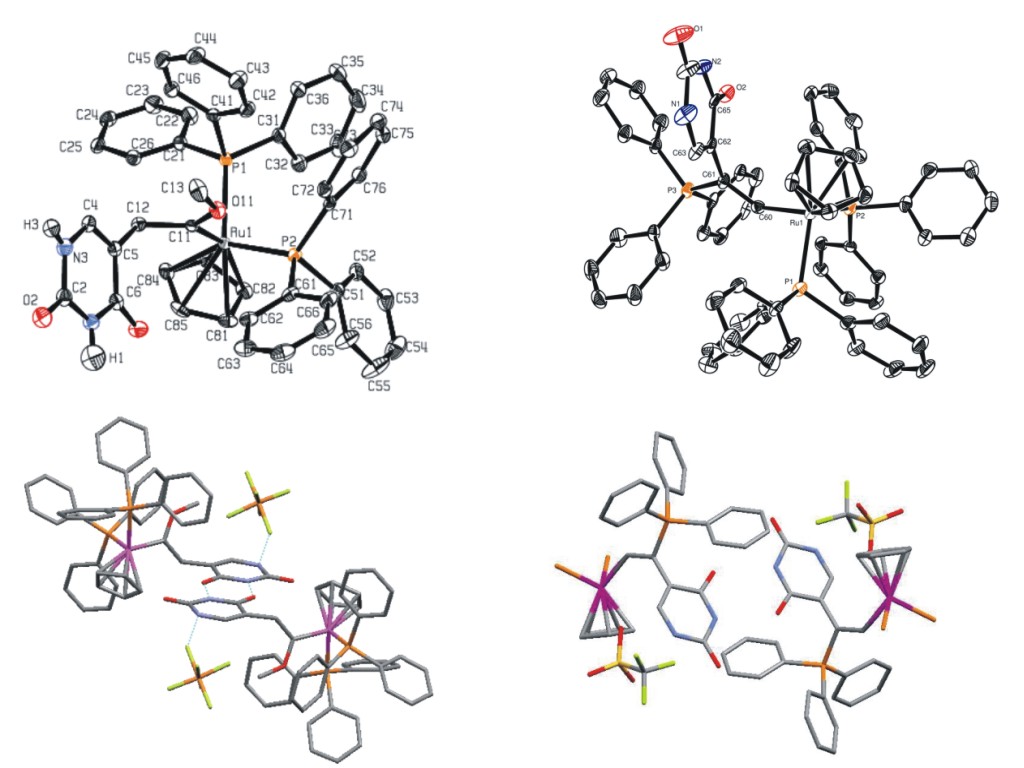
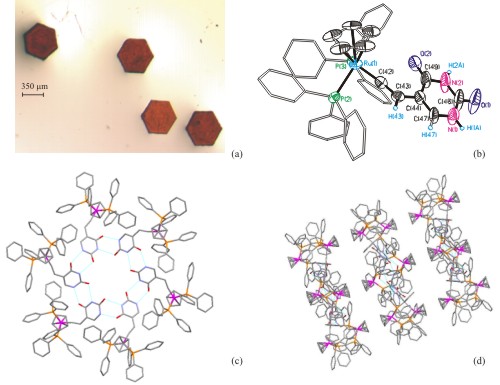
The non-covalent interactions between the nucleobases with DNA represent perhaps one of the most important examples of molecular recognition. We have been interesting in exploiting these interactions to direct the assembly of novel architectures based on organometallic complexes. For example, we have shown that the uracil-substituted alkyne 1 maybe employed as a precursor to ruthenium carbene complexes, such as 2 and vinyl complexes, such as 3. The critical part of the synthesis is that the organometallic precursor is highly selective for the alkyne functionality of 1 so that hydrogen bonding may be maximised. Our current research goals are to expand these studies, for example by varying ligand sets and nucelobase, so that more complex solid-state structure may be prepared. The most striking example we have prepared to date is a ruthenium complex which self-assemebles to form a hexameric array - the self assembly being controlled by hydrogen bonding interactions. |
|
 |
|
| 1 wwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwwww2 | |
 |
|
Low-coordinate Phosphorus Chemistry
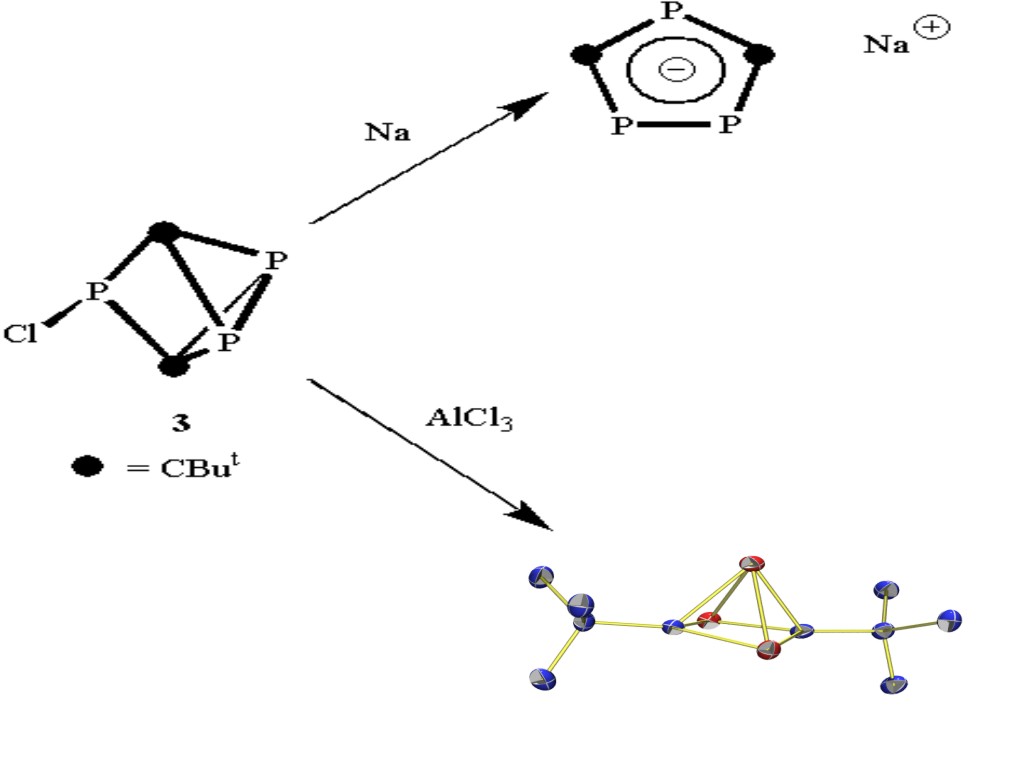
In recent years there has been considerable interest in the isolobal and isoelectronic relationship between phosphorus and carbon and the preparation by Becker of the first kinetically stabilised phophospalkyne has led to a vast expansion of this field. We are interested in utilising phosphaalkynes as fundamental building blocks for the synthesis of new organophopshours compounds. For example, we have shown that phosphaalkynes may act a h2(4e) donor ligands within the coordination sphere of transition metal complexes.More recently, we have employed the interesting triphosphorus cage compound 3 as a precursor to the planar anion 4 (isoelectronic with C5H5-) and also the cationic nido-cluster 5. Compound 5, which is isoelectronic with C5H5+, represents one of the first examples of an organophosphorus compound which obeys Wades Rules.The chemistry of these compounds is being explored in
collaboration with Professor Michael Green and Dr Chris Russell at the
University of Bristol and with the theoretical group of Dr John McGrady
(University of Glasgow)
|
|
|
|
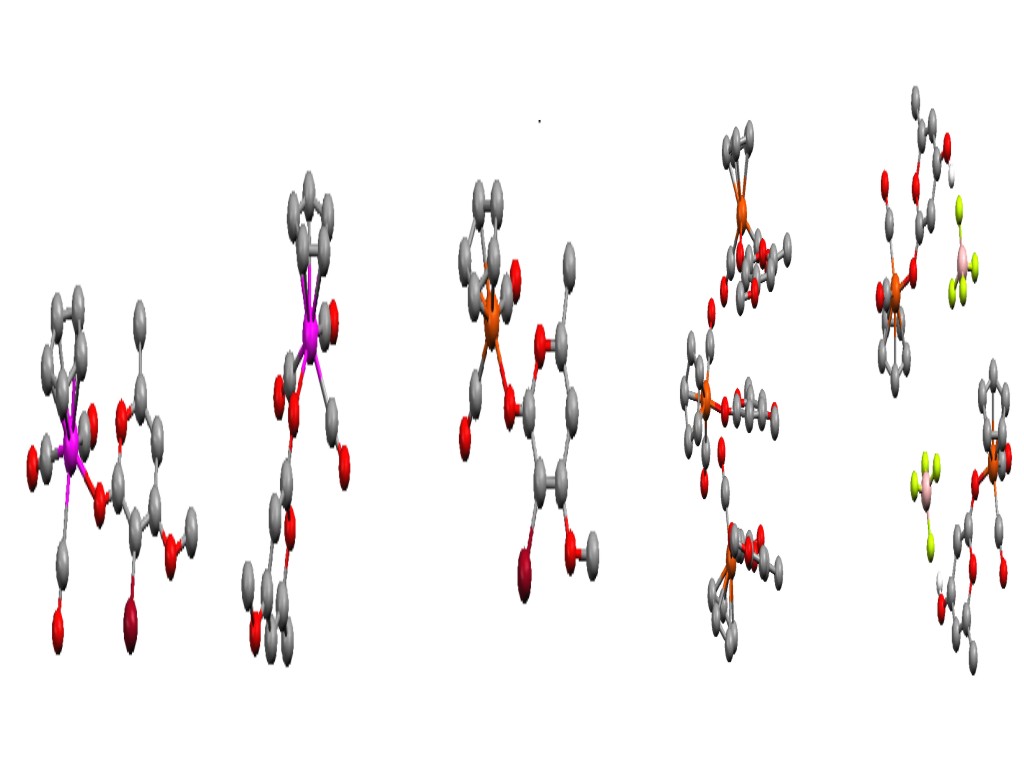
Carbon Monoxide-Releasing Molecules.
Despites its reputation as a poisonous gas, Carbon-Monoxide has recently
been identified as playing an important biological role. Indeed, CO
has been shown to act as an effective theraputic agent for the treatment
of a range of conditions. In particular, at low concentrations, CO has
been shown to be a vasorelaxant and it also exhibits anti-inflammatory
properties. CO gas does not, however, provide a long-term therapeutic
solution to exploiting these beneficial properties. Therefore, in collaboration
with Dr Ian Fairlamb and Anne Duhme Klair (York) and Dr Roberto Motterlini
(Northwick Park Hospital), we have investigated a range of metal carbonyl
compounds designed to liberate CO under biological conditions. We have
therefore investigated the synthesis of a range of Carbon Monoxide-Releasing
Moleceules (or CO-RMs) and investigated their mechansim of action.
|
|
 |
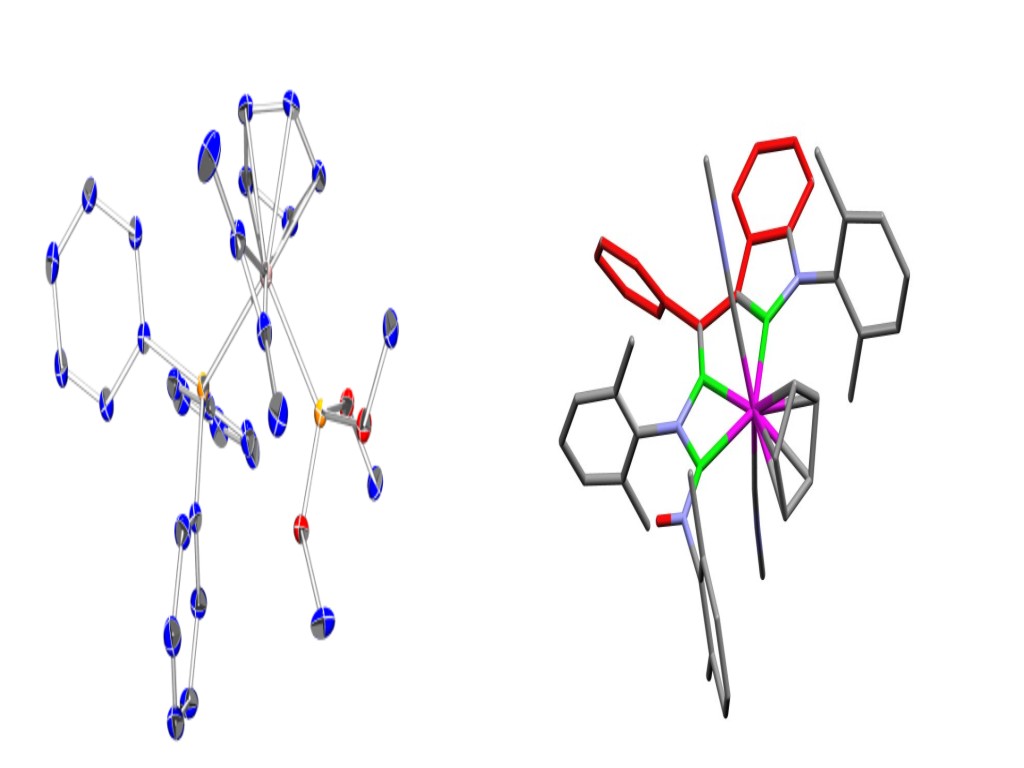
Reaction and Mechanisms of Alkyne Complexes
We have a considerable interesting in the development of the chemistry of molybdenum complexes containing alkyne ligands which donate four-electrons to the metal centre. For example, we have devised new routes to these species and also explored their often fascinating chemistry. In particular we have examined how isonitriles and alkynes may be combined within the coordination sphere of the metal to give complex organic ligands such as the highly substituted indole shown on the far right. Current work is focused on the studies of fundamental mechanistic process at the molybdenum centre. |
 |
|
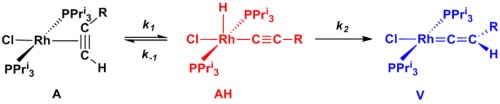
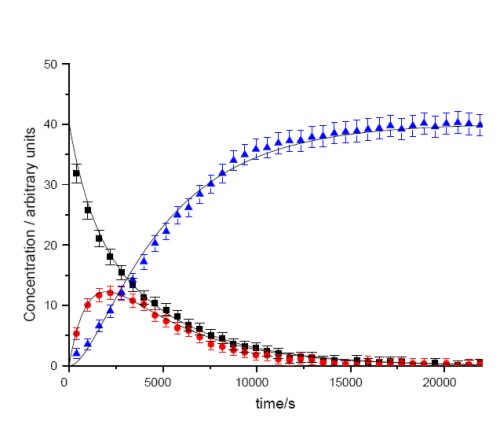
We have also investigated the mechansim by which a rhodium vinylidene complexes are formed. Using NMR spectroscopy, we have monitored the formation of alkyne, alkynyl hydride and vinylidene complexes. A analysis of the data demonstrates that the reaction proceeds in a unimolecular fashion and that the formation of the vinylidene is essentially irreversible. |
 |
|
|
|
||