 d
d
A multicentre randomised controlled trial was carried out to compare the effectiveness of nasal diamorphine spray with intramuscular morphine for analgesia in children and teenagers with acute pain due to a clinical fracture (BMJ 2001; 322: 261-265). In the emergency departments of eight UK hospitals, patients aged between 3 and 16 years presenting with a clinical fracture of an upper or lower limb were randomised to one of the treatments. The main outcome measures were patients’ reported pain using the Wong Baker face pain scale, ratings of reaction to treatment of the patients and acceptability of treatment by staff and parents, and adverse events. 404 eligible patients completed the trial (204 patients given nasal diamorphine spray and 200 given intramuscular morphine).
The authors stated that ‘The distribution of pain scores for the spray group was lower than that for the intramuscular group at 5 (P=0.04), 10 (P=0.003), and 20 minutes (P=0.002) after treatment, but no different after 30 minutes (P=0.20)’.
Question 1. What can we conclude about pain score after 10 minutes?
The pain scores after 30 minutes were as follows:
| Pain score | Nasal diamorphine group | Intramuscular morphine group | ||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| 1 | 54 | (28) | 48 | (25) |
| 2 | 65 | (34) | 61 | (32) |
| 3 | 36 | (19) | 42 | (22) |
| 4 | 24 | (13) | 23 | (12) |
| 5 | 7 | (4) | 11 | (6) |
| 6 | 5 | (3) | 8 | (4) |
Question 2. Is it correct to conclude that pain scores were no different after 30 minutes?
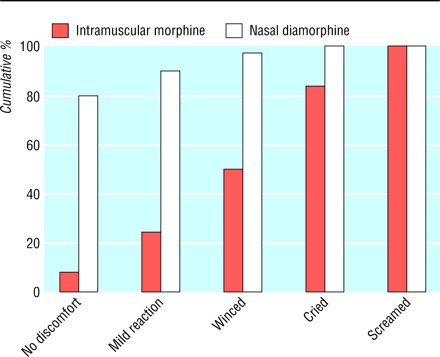
The following graph was used to present the data on reaction to the treatment:
Question 3. What kind of variable is reaction to the treatment? What does this graph actually show?
Question 4. How could the graph be improved?
To IAPT index.
To Martin Bland's M.Sc. index.
This page maintained by Martin Bland.
Last updated: 23 January, 2009.