Nano- and Biomaterial Physics
Group
Nano- and Biomaterial Physics
Group
• Home
• People
• Research
• Methods
• Publications
• Funding
• Jobs
• Gallery
• Contact
Methods and Tools
|
Liquid
cell transmission
electron microscopy
A
novel sample holder (Protochips Inc.) for transmission electron
microscopy (TEM) allows
for the imaging of objects in liquid environments such as minerals
forming from supersaturated ionic solutions or biogenic materials that
undergo significant changes when dried or frozen to be imaged in TEM. A
schematic shown in Figure 1
This
versatile technique gives access to many aspects of liquid phase
processes with an unprecedented spatial resolution of less than 1 nm,
especially if scanning TEM (STEM) is used for imaging. We are
using this technique to study the dynamics of the
formation of minerals such as calcium carbonate
or hydroxyapatite from solution. An important aspect in this context is
the role of amorphous precursor phases for the growth rates, polymorph
selection and shape evolution. Moreover, this method allows for the
investigation of biological materials such as bacteria in liquids,
which we aim to develop.
|
|
Atmospheric scanning
electron microscopy
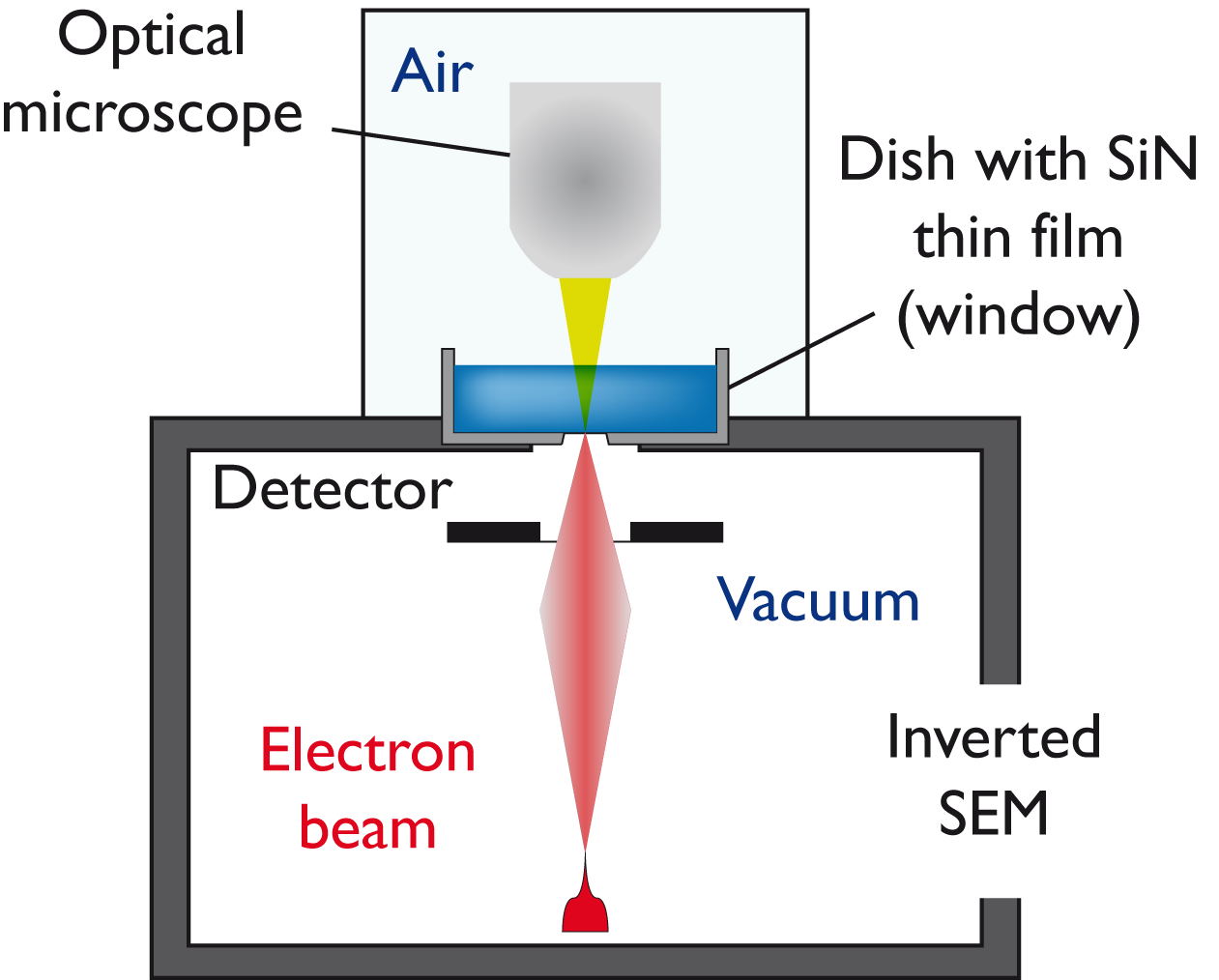
The
examination of processes in liquids using a scanning electron
microscope (SEM) offers a huge scope of questions to be addressed
regarding mineral formation in liquids. Such a unique system (JEOL
Clairscope available at the Bioscience
Technology Facility), is available in York and consists of an
inverted SEM
combined with a confocal optical microscope and allows for the imaging
of processes and objects in liquids using back-scattered electron
detection as shown in Figure 2. The
object remains in liquid and under atmospheric condition
during the observation. Our studies focus on the precipitation from
supersaturated solutions, and in particular the effect of the presence
of organic additives - important in many biological systems - on this
process. Results of
these investigations have been published in the Journal of Structural
Biology (Journal
of Structural Biology 2013).
|
|
Atomic
level strain
analysis of core/shell nanoparticles
Aberration
corrected transmission and scanning transmission electron microscopy
offer exciting opportunities for the study of materials with a spatial
resolution
of better than 1 Å. We use this tool (JEOL 2200 FS) to investigate the
oxidation of iron nanoparticles deposited by a cluster source. The
unprecedented resolution enables the detailed study of strain in the
oxide shell formed around a cubic iron nanoparticle. Using atomic level
strain-state analysis it was possible to determine the strain within
the oxide layer forming on the iron core as shown in Figure 3. It revealed that extreme
strains can exist on the nanoscale due to the fact that dislocations,
which would normally relax this strain in bulk systems, are
energetically unfavorable at these length scales and hence strains of
approx. 10% can be sustained. This effect was found to be important
with e.g. in the accelation of otoxidation process in iron
nanoparticles (Nature
Materials 2013).
|
|
Raman
microscopy
We
are using a Horiba XploRA Raman Microscope to study the structure of
minerals (e.g. carbonates and phosphate), mineral/organic composites
(e.g. corals, bones and teeth) and organic microfibers such as collagen
and cellulose. The system is equipped with three laser sources (with
wavelengths of 532 nm, 633 nm, 785 nm) allowing for material specific
analyses.
|
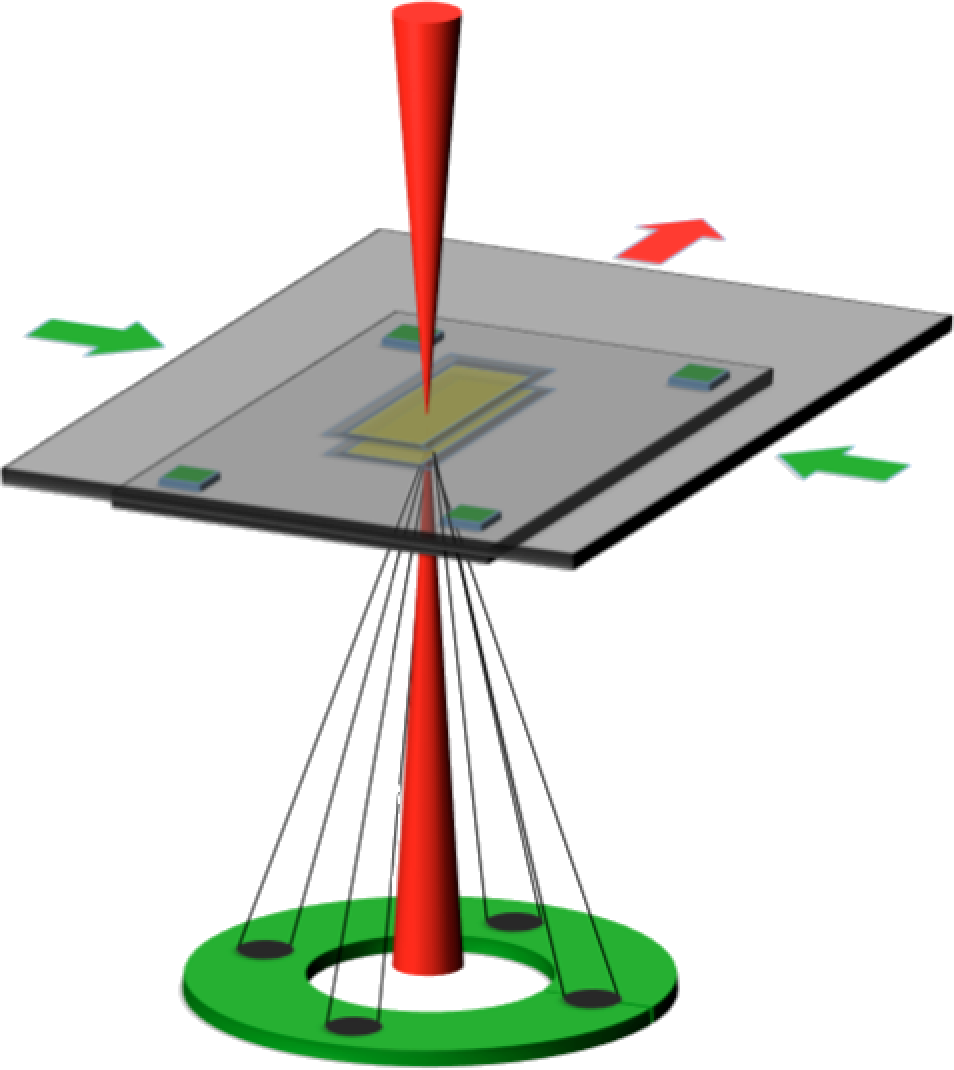 Figure 1:
Schematic of the liquid-cell transmission electron microscopy setup
allowing for imaging of liquids through a pair of silicon nitride
membranes.
|
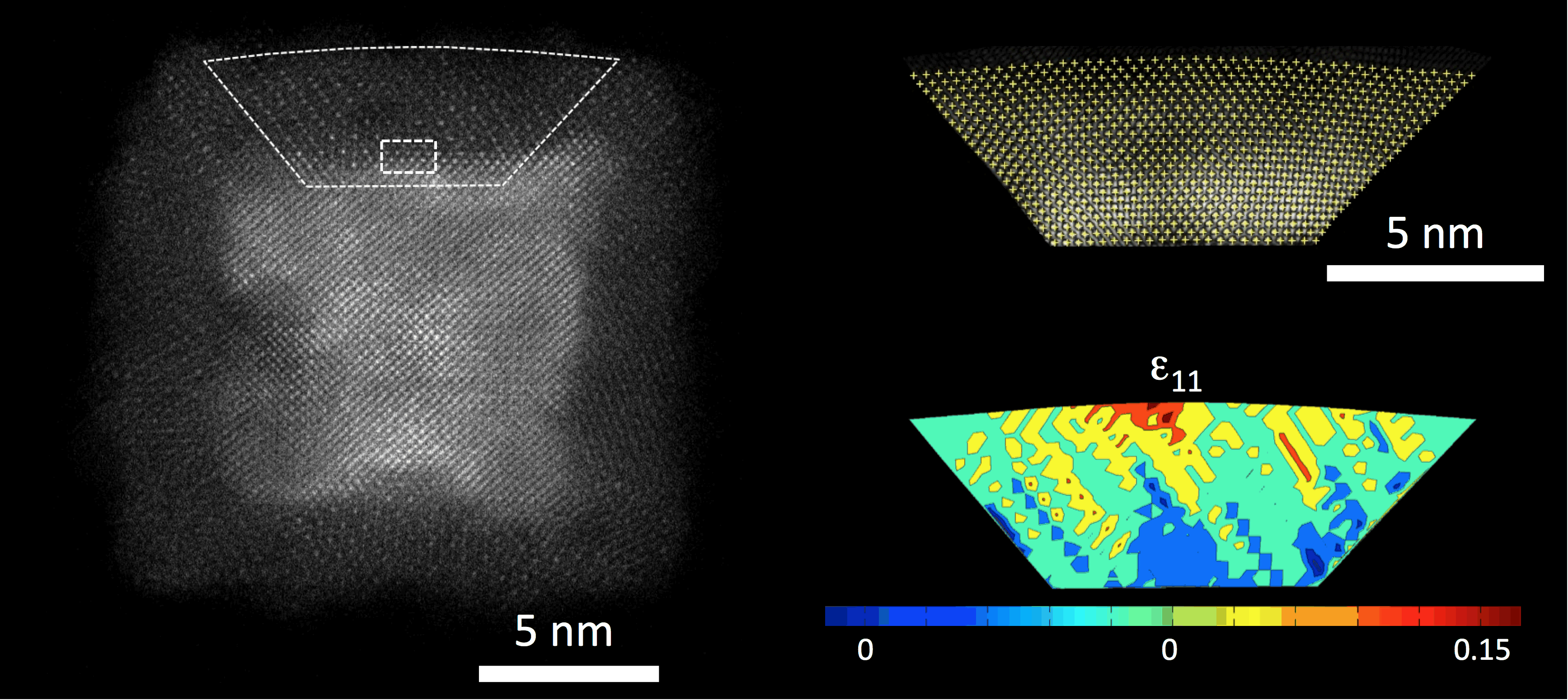 Figure 3:
Atomic-level strain state analysis of an iron nanoparticle which has
undergone oxidation in air. Figure 3:
Atomic-level strain state analysis of an iron nanoparticle which has
undergone oxidation in air. |