Nano- and Biomaterial Physics
Group
Nano- and Biomaterial Physics
Group
• Home
• People
• Research
• Methods
• Publications
• Funding
• Jobs
• Gallery
• Contact
Research Highlights
Studying the precipitation of minerals with and without additives
The
formation of mineral/organic composites such as shells in marine organisms as well as teeth or
skeletons is largely determined by the presence of organic additives
such as proteins or polysaccharides and
the interaction of the ions in solution with these additives prior to precipitation and mineral growth. Novel
electron microscopy techniques such as liquid
cell transmission
electron microscopy or atmospheric scanning electron microscopy
allow
for the in situ investigation
of precipitation processes with unprecedented spatial resolution. In
collaboration with the group of John S. Evans (NYU) we have performed
studies of calcium carbonate formation in the presence of the protein
AP7 extracted from the nacre of Haliotis
Rufensis
known to play an active role in the nucleation and crystal growth. Movie 1
shows the agglomeration and growth of the mineral/protein
composite. The electron beam can trigger the crystal growth. It is
found that if the electron dose is reduced, the crystal phase dissolves.
Movie 2 shows the impact of polyacrylic acid on the growth of calcite crystals from solution using an atmospheric SEM. As it can be observed, a transient - most likely amorphous - phase is formed prior to the onset of crystal growth after which the transient phase dissipates. These studies also show the formation of a depletion zone around the growing crystal indicating a reduction of calcium ion concentration in the vicinity of the growing crystals into which the ions are incorporated (Journal of Structural Biology 2013). With this technique we could also visualize the dynamics of the growth of the carbonate polymorph aragonite in the presence of ethanol (see Movie 3).
Movie 2 shows the impact of polyacrylic acid on the growth of calcite crystals from solution using an atmospheric SEM. As it can be observed, a transient - most likely amorphous - phase is formed prior to the onset of crystal growth after which the transient phase dissipates. These studies also show the formation of a depletion zone around the growing crystal indicating a reduction of calcium ion concentration in the vicinity of the growing crystals into which the ions are incorporated (Journal of Structural Biology 2013). With this technique we could also visualize the dynamics of the growth of the carbonate polymorph aragonite in the presence of ethanol (see Movie 3).
Nanoparticles for
biomedical applications
In
collaboration with Chris Binns (University of Leicester) we investigate
cluster deposited nanoparticles aimed to be used as nanovectors for
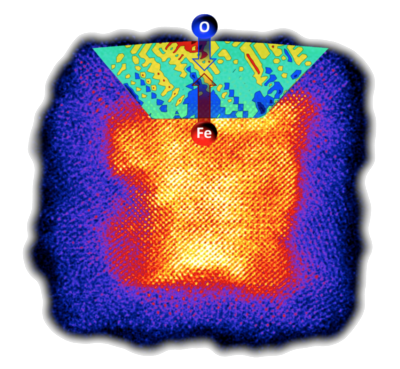
theranostic applications. Figure
1 shows a
colour-coded
high-resolution
scanning transmission electron micrograph of a cluster deposited iron
particle which has undergone oxidation after exposure to air. The image
was taken using an aberration-corrected STEM as part of a collaboration
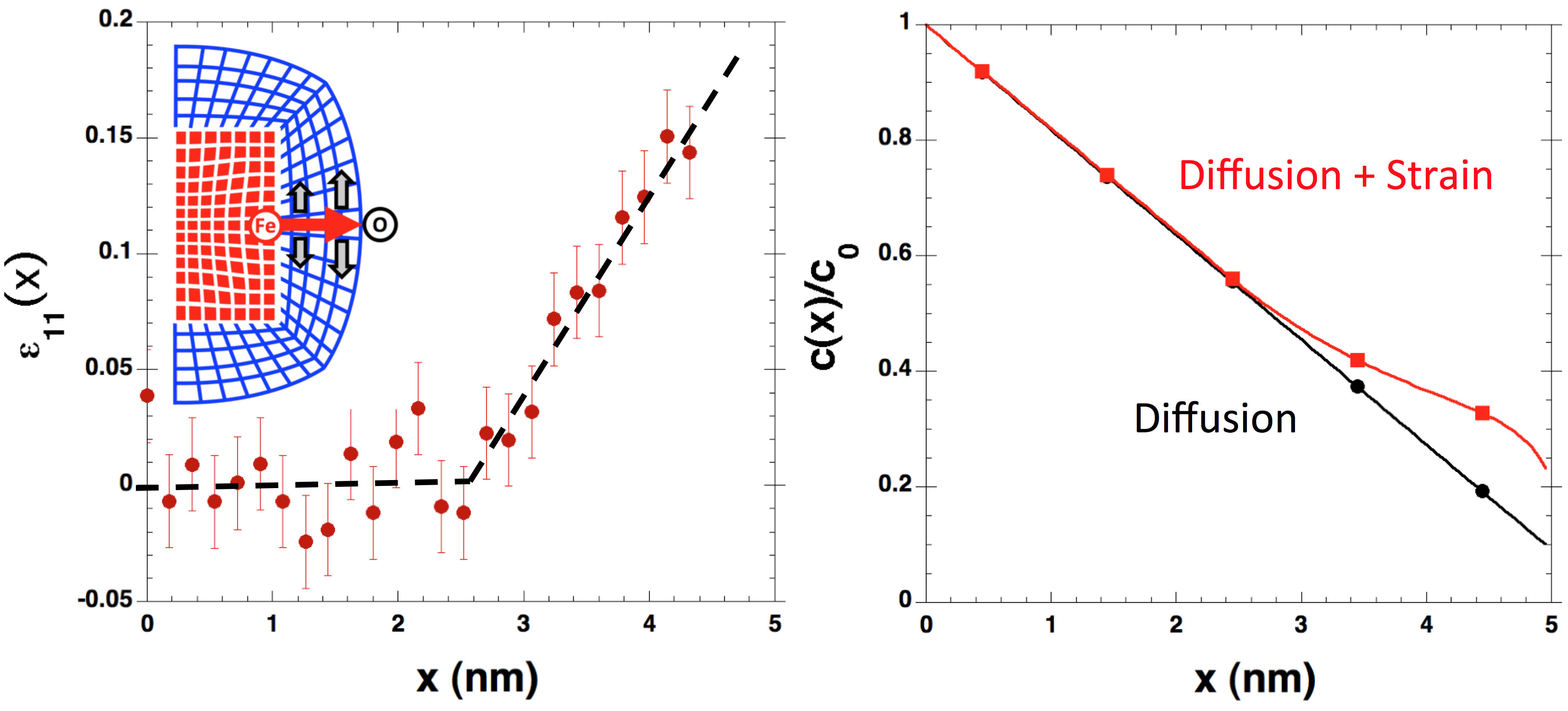
with the University of Illinois. A detailed analysis of the lattice
strain on the atomic level reveals a significant strain in the oxide
layer responsible for an enhancement of the oxidation rate (Nature
Materials 2013).
Correlation between
diurnal growth bands and crystal microstructure in corals
In
this study we show that new insights can be gained on the nano- and
microstructure of corallites by TEM investigation of large-scale (15 x
30 µm) FIB lamellae from adult and juvenile scleractinian coral skeletal
specimens as shown in Figure 2. By leaving the FIB
prepared lamella
within the coral skeletal context (no lift out) the lamella is
mechanically more stable and durable while being directly comparable to
the larger scale (several tens of microns) not ion-milled skeletal
areas by optical analysis. Thus we could identify a crystallographic
evolution from a center of calcification outward over acicular and
granular (daily) bands. We draw a parallel to the diurnal
photosynthetic cycle of the zooxanthellae in symbiotic corals that
control the levels of oxygen and carbon availabilities, both recognized
as significant drivers of coral calcification processes. The transport
of large amounts of glycerol by zooxanthellae and its potential impact
on coral calcification was also discussed. This process could play a
role in the specific alignment of the aragonite crystals as was
previously demonstrated by synthesis experiments with OH group
containing additives and the TEM investigation of these precipitates.
The juvenile Acropora millepora specimen also showed the large acicular
crystals interrupted by thin porous bands, but lacked the
nanocrystalline phase, which may be linked to the absence of
zooxanthellae and thus the typical daily cycle (Journal
of Structural Biology 2013).
Assembly pattern of nanocrystals in spicules of Rhabdosphaera Clavigera
Coccoliths
are micrometer scale disks build up from single crystal calcite units,
produced by unicellular marine algae, belonging to the phylum
Haptophyta. The complex biomineral structure exhibited by these
organisms, as depicted in Figure 3, shows little
resemblance to their
geological or inorganic equivalents and is impossible to reproduce
synthetically. This implies a stark control by the organism over
crystallographic orientation and overall morphology to create
functional structures and motivates a detailed study of the
microstructure with the aim to unveil fundamental aspects of
nanocrystal formation and assembly in biological systems.
Mineralisation of
bones and teeth
Recently
we have commenced to investigate the mineralisation patterns in bones
and teeth in collaboration with Paul Genever (Biology, York) and Steve
Weiner (Weizmann Institute, Israel). Aim is to use electron microscopy
based techniques to obtain a detailed insight into the correlation
between organic phases and the hydroxyapatite phase formed by the bone
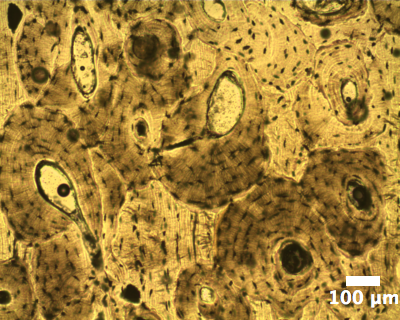
and tooth forming cells. Figure 4 shows the optical
micrograph of human
bone sample that had been sliced and thinned down to approx. 10 µm. it
clearly shows the osteons and bone cells responsible for the
mineralisation.
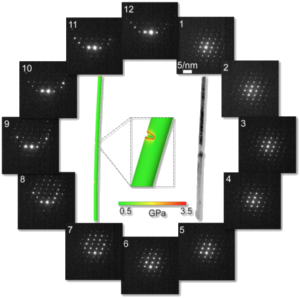
Anisotropy and lattice distortions in calcite nanowires
It
is believed that the formation of minerals in biological systems is
strongly determined by the early stages of growth, usually occurring in
confinements e.g. created by cells. Therefore, we are particularly
interested in the properties of nano-structures such as calcite
nanowires grown in confinement. Using electron diffraction in
conjunction with finite element calculations (performed by the group of
Dr Dorothy Duffy at UCL) we identified an important correlation between
the anisotropic crystal structure and lattice distortions such as twist
and bending for calcite nanowires of several µm in length and less than
100 nm in diameter (see Figure 5).
Collaborations
We are collaborating with leading scientists in the UK:
James Elliott, University of Cambridge
John Harding, University of Sheffield
Nicola Allison, University of St. Andrews
We are also working with leading international research groups all over the world e.g.:
Helmut Cölfen, Chemistry, University of Konstanz, Germany
Denis Gebauer, Chemistry, University of Konstanz, Germany
John S. Evans, New York University, New York, US
Roie Yerushalmi, Hebrew University, Jerusalem, Israel
Laurie Gower, University of Florida, US
Anna Tampieri, Faenza, Italy
Martin Saunders, University of Western Australia, Australia
| Movie
1: Precipitation of calcium
carbonate
in the presence of the
nacre-derived protein AP7 in situ
observed by liquid cell scanning
transmission electron microscopy. Movie is accelerated by a factor of 30. |
Atmospheric SEM


Movie
2: Precipitation of calcium
carbonate
in the presence of poly-acrylic acid (PAA) in situ observed by atmospheric
scanning electron microscopy (ASEM). The video is accelerated by a
factor of 30.
Atmospheric SEM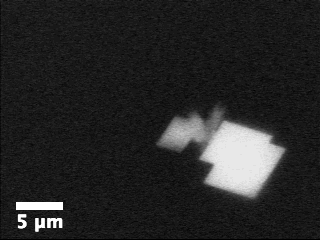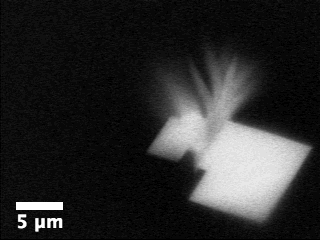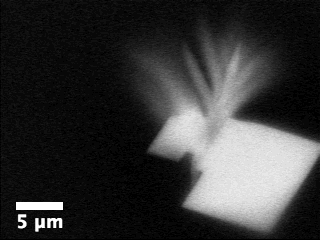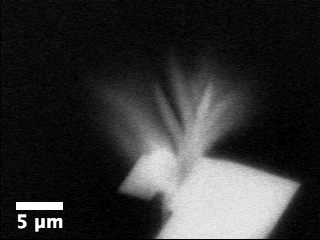
Movie
3: Growth of aragonite on calcite in the presence of ethanol as
imaged by ASEM.
|
|
Nanocrystal assembly in corals
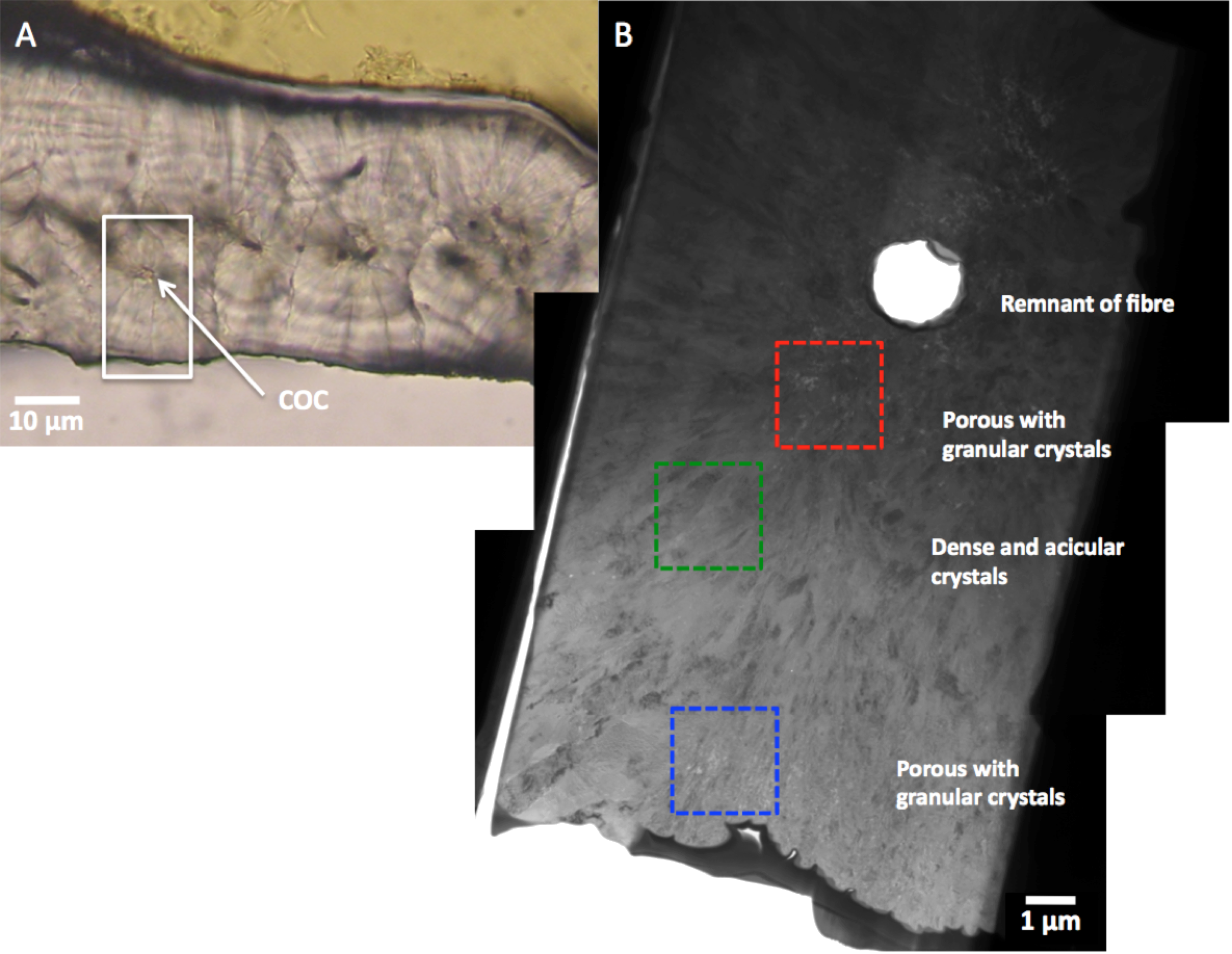 |
| Figure 2: Optical micrograph and
transmission electron microscopy image
showing details of a spherulite in the sidewall of the coral skeleton. |
| Figure 3: SEM image of a
spicule
of the coccolithophore Rabhdosphaera
Clavigera. |
| Figure 4: Optical micrograph (in transmission) of human bone osteons. |
| Figure 5: Electron microscopy and finite element analysis of calcite nanowires. |