1. New avenues in anti-viral drug design
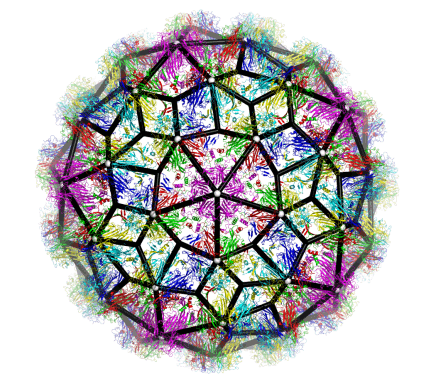
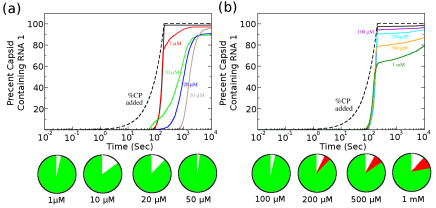
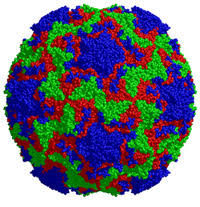
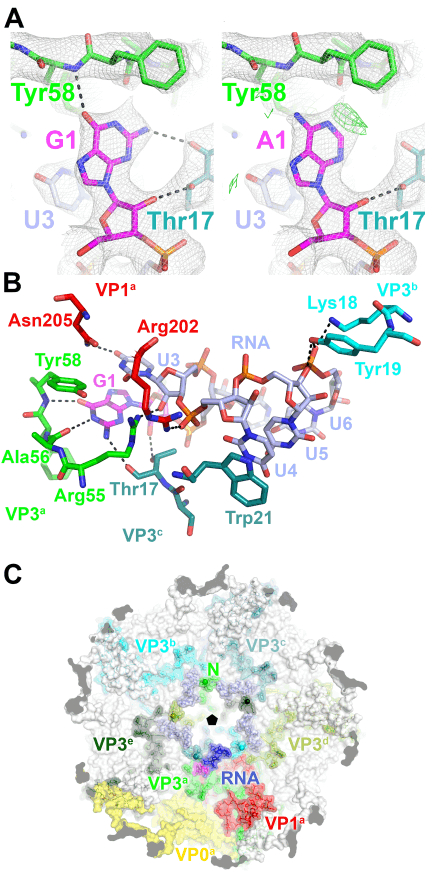
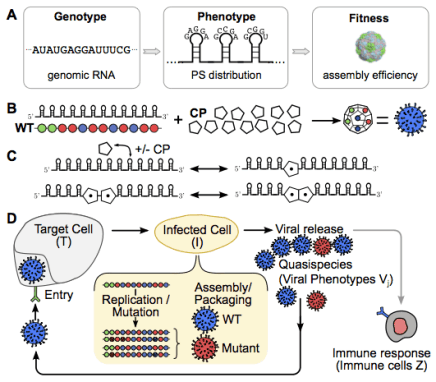
The discovery of an RNA-encoded assembly instruction manual that is mediated by signals in the genomic viral RNA has opened up novel drug targets. We developed efficient tools for the identification of these signals and their binding sites on the capsid protein, and characterised the molecular details of these interactions. This enabled us to identify new drug targets, and we hold two patents (“Anti-viral Therapy” PCT/ GB2014/052696; “Viral Packaging Signals” GB1618094.5) for novel anti-viral strategies against these viruses. The spectrum of viruses covered by these patents includes Hepatitis B (HBV), a retro-transcribing DNA virus that packages its genome initially as a pre-genomic RNA. Using a bespoke high-throughput screen against >22,000 potential RNA binding ligands, in collaboration with the US NIH at Frederick, we identified >60 specific “hits” to the generic HBV genomic sites contacting the viral protein shell. These small molecular weight compounds were then screened for toxicity and assembly inhibition in collaboration with Professor Marcus Dorner, Imperial College, London. This identified 9 compounds with significant initial anti-viral activity, and we are now assaying their effects against virus in collaboration with colleagues at the NIH, who have also filed a patent, led by Dr Stuart LeGrice, NIH (“Methods of treating HBV”, U.S. Patent Application #62/685,145, 2018), that includes my experimental collaborator Peter G Stockley and myself as inventors. We have similar “hits” from the screen using the parechovirus genome targets.
We are in discussions with industrial partners to exploit our invention commercially for HBV, and have plans to follow similar routes for other viruses.
2. New opportunities in synthetic vaccines and gene therapy
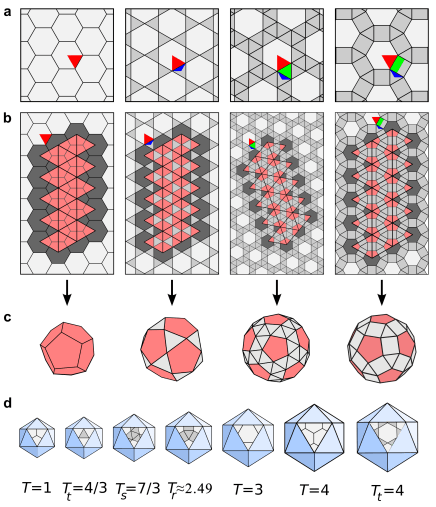
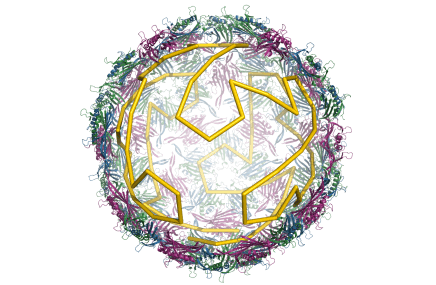
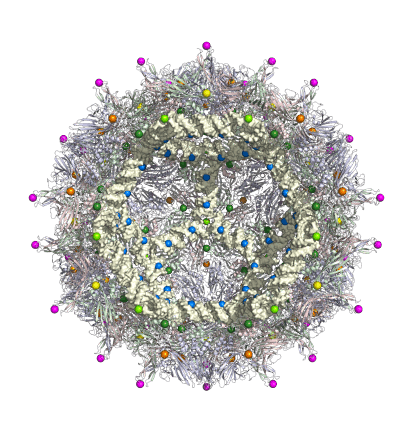
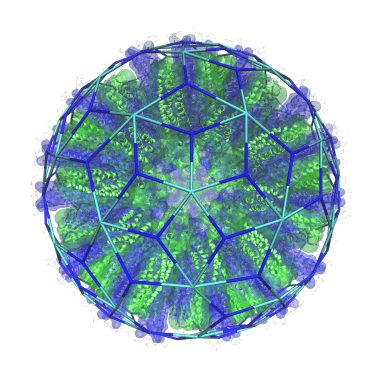
The mechanistic understanding of how packaging signals contribute to efficient formation of viral particles provides a basis to repurpose viral components in the design of virus-like particles (VLPs). We have demonstrated that the essential features of the RNA-encoded assembly manual can be used to enhance the efficiency of assembly around non-viral RNAs (see our patent “Virus-Like Particles” GB1708709.9), and we are in the process of extending this idea to major pathogens for which there are currently no vaccines, but for which novel forms of intervention are urgently required.
Virus-like particles are also of interest as transport vehicles in gene therapy. I am currently developing algorithms that support the development of technologies that render the assembly of VLPs vastly more efficient. Overcoming the assembly efficiency bottleneck is essential in order to make sufficient VLPs for successful treatment in patients.